The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. Program bmw x5 garage door opener. Therefore, the number of electrons in neutral atom of Nickel is 28. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other (Z – 1) negative electrons in the atom. Number of protons in an atom of Boron. Number of neutrons in an atom of Boron. Number of electrons in an atom of Phosphorus. Mass number of Phosphorus.
:max_bytes(150000):strip_icc()/GettyImages-523446050-5897be0a5f9b5874ee7c9fa6.jpg)
Answer: mass
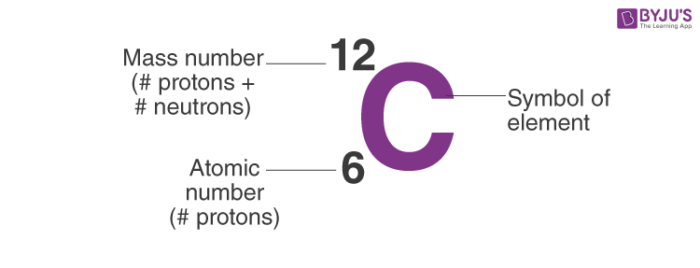
Atomic Number: Number of protons and it is also the number of electrons in an atom of an element. Element’s Symbol: An abbreviation for the element. Elements Name Atomic Mass/Weight: Number of protons + neutrons. An atom with three protons is a lithium atom, an atom with five protons is a boron atom, an atom with six protons is a carbon atom. The list goes on. Since an atom of one element can be distinguished from an atom of another element by the number of protons in its nucleus, scientists are always interested in this number, and how this number.
Number Of Protons In An Atom’s Nucleus Determines Its
Most relevant text from all around the web:
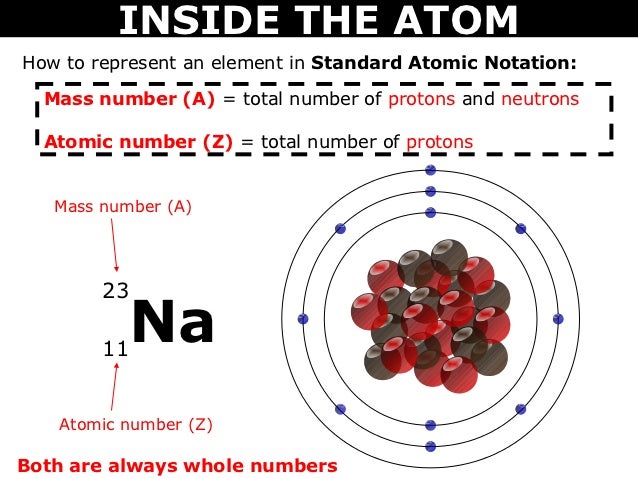
The number of protons plus neutrons in an atom is its ________ number. The atomic number or proton number (symbol Z) of a chemical element is the number of protons found in the nucleus of every atom of that element. The atomic number uniquely identifies a chemical element. It is identical to the charge number of the nucleus. In an uncharged atom the atomic number is also equal to the number of electrons. The sum of the atomic number Z and the number of neutrons N gives the mass numberA of an atom. Sin… The mass number (symbol A from the German word Atomgewicht [atomic weight]) also called atomic mass number or nucleon number is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus.It is approximately equal to the atomic (also known as isotopic) mass of the atom expressed in atomic mass units.Since protons and neutrons are both baryons the mass number .. By definition any two atoms with an identical number of protons in their nuclei belong to the same chemical element. Atoms with equal numbers of protons but a different number of neutrons are different isotopes of the same element. For example all hydrogen atoms admit exactly one proton but isotopes exist with no neutrons (hydrogen-1 by far the most common form also called protium) one neutron (deuterium) two neutrons (tritium) and more than two neutrons. The known elements form a set of atomi… Mass number - Wikipedia Atomic number - Wikipedia Atom - Wikipedia Atomic number - Wikipedia The number of protons an atom has defines what chemical element it is this number is sometimes called its atomic number. For example hydrogen has one proton and sulfur has 16 protons. Because the mass of neutrons and protons is very similar and the mass of electrons is very small we can call the amount of protons and neutrons in an atom its atomic mass . mass number (symbol: A) which is the sum of the number of protons and num..
Number Of Protons In An Atom Of Iron

Disclaimer:
The number of protons in an atom equals the number of what?
:max_bytes(150000):strip_icc()/atomic-mass-and-mass-number-606105_v1-80df956ab98440bc9969531d1bb6c874.png)
1 Answer
The number protons equals the number of electrons in a NEUTRAL atom.
Explanation:
Protons are conceived to be positively charged, massive nuclear particles. Electrons are conceived to be particles of negligible mass that orbit the nuclear core. Clearly, for a neutral atom, the number of electrons must equal the number of nuclear protons. The number of nuclear protons,
Now an atom can gain or lose electrons to form a negatively or positively charged ion; it cannot lose nuclear protons because it that case the identity of the nucleus changes.
Related questions
